usp class vi compliant
USP Class VI Compliant Materials for Medical and Pharmaceutical Products Meets USP Class VI requirements for use in medical and pharmaceutical applications. Final Report Date June 18 2008 COMPLIANCE 21 CFR Part 58 Good Laboratory Practice for NonClinical Laboratory Studies MANAGEMENT OF THE STUDY Performing Laboratory Toxikon Corporation 15 Wiggins Avenue Bedford MA 01730 Sponsor DSM Somos.
Dursan Passes Usp Class Vi Testing Why Is That Important
Compliance to USP Class VI is often requested by users in the biopharmaceutical and medical industries.
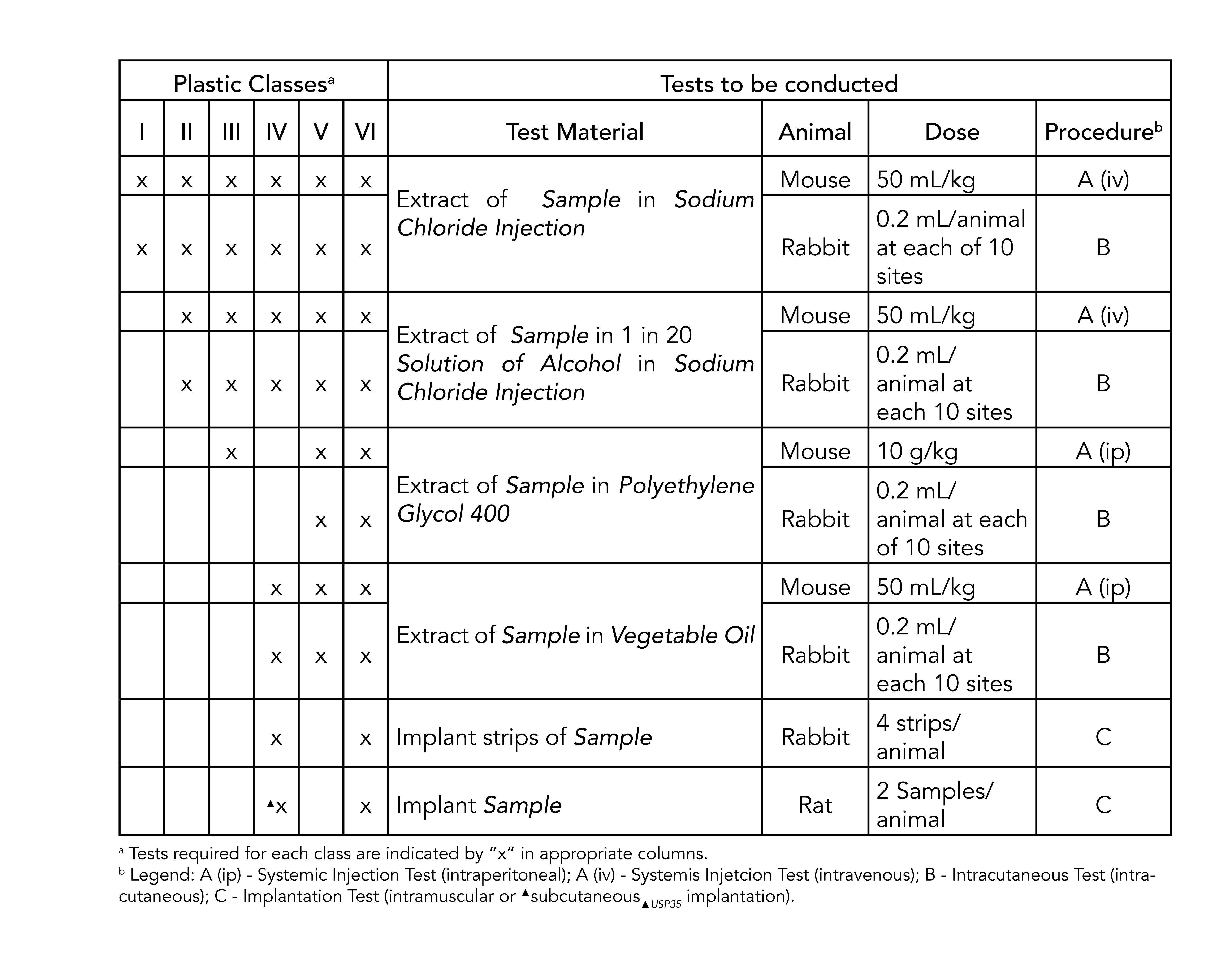
. There are six classes VI being the most rigorous. SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. Tests of the provided material samples passed all requirements and have been approved for.
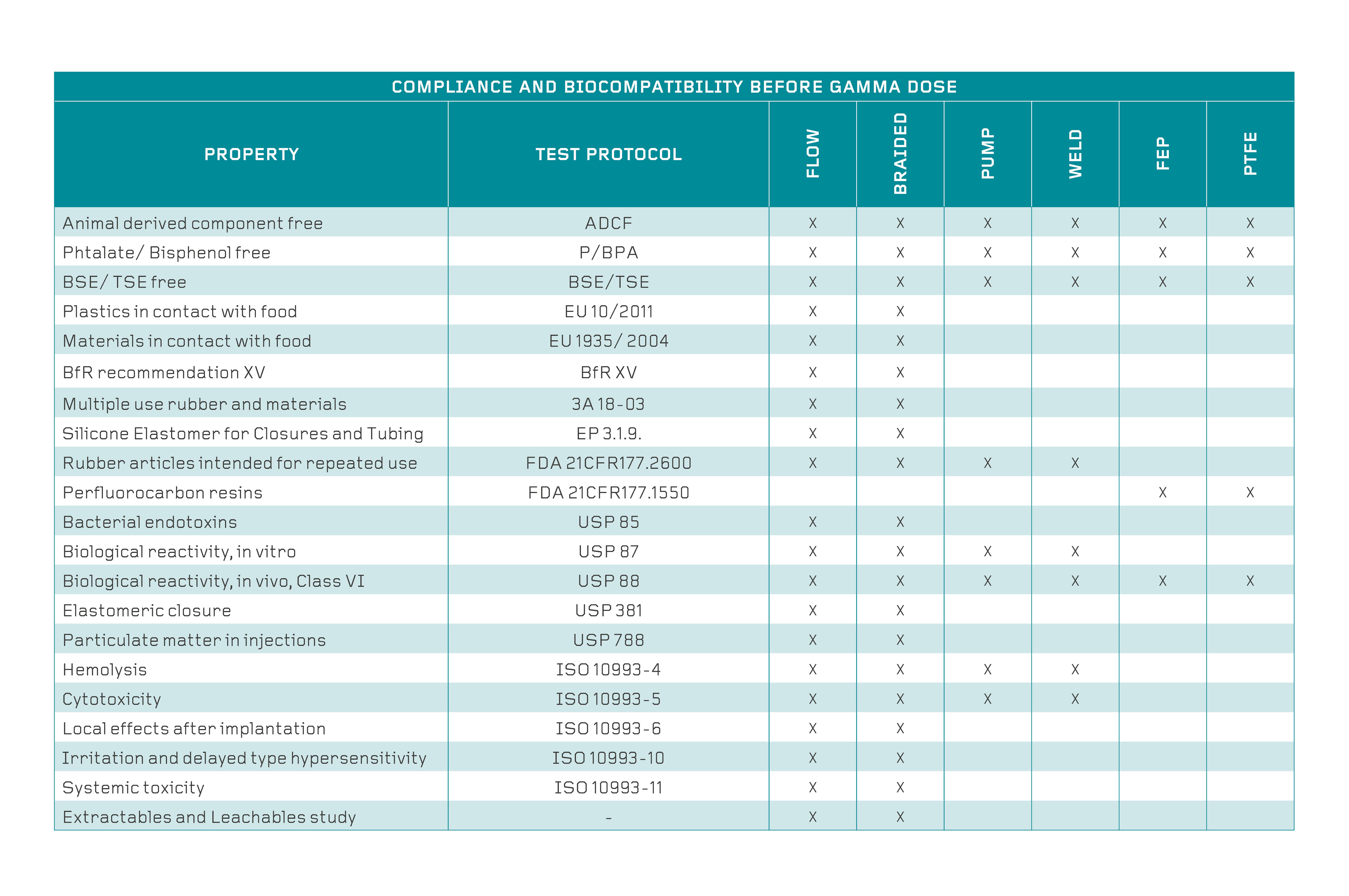
Sil 714001 USP class VI Silicone 1 70 Yes transl. EPFEPerfluoroelastomer Seals Special RubberFabrications. Suitability under USP Class VI is typically a base requirement for medical device manufacturers.
Request a Quote Now. USP Class VI Certificate of Compliance. USP Class VI and ISO 10993-1 Information.
Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements. As one of the most widely used methods VI forms part of six different classes with this being the most thorough. Sil 714002 USP class VI Silicone 1 70 Yes transl.
Compliance to USP Class VI is often requested by end users. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. This form of testing is designed to certify that no harmful reactions or long-term issues are caused to the body by chemicals that are released or leached from plastic materials.
USP Class VI materials meet the most stringent requirements and include silicones that pass a systemic toxicity test an intracutaneous test and an implantation test. Professional Plastics General Product Range Brochure. What does compliance with USPNF standards mean.
Testing for compliance involves an assessment of the effects of the material and extractables on tissue. Graco Company have been tested for compliance to USP Class VI 70C plastic. RoHS a European Union Directive restricts the use of certain substances but manufacturers also need to know whether all the ingredients in a medical silicone are made of compliant materials.
Yet some suppliers that use compliant ingredients may still not be able to guarantee a compliant end-product. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials. Specially formulated for long term sealing.
An article of commerce that is recognized in the USPNF complies with USPNF standards when it meets all of the requirements stated in the articles monograph applicable General Chapters and the General Notices with monograph requirements superseding those of the General Chapters. Biocompatibility Information for Materials. Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and surgical equipment.
USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. ADI-free certifies that the raw materials used in production of the elastomer contain no Animal Derived Ingredients ADI. When evaluating a new product many of our customers immediately jump to USP Class VI approval tests.
Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials. The USP defines six plastics classes from class I to class VI with class VI being the most rigorous and most frequently requested certification. Compounds made without animal-derived ingredients BSETSE concerns.
A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing. 157 Charles Colman Boulevard Pawling NY 12564. United States Pharmacopeia USP 26 NF21 2003 Class VI.
Standards are published in the US Pharmocopeia and the National Formulary USP NF. Master Bond systems are very versatile and can be used for both disposable and reusable medical devices. A number of our plastic materials are ISO-10993 or USP Class VI capable.
Specialty Silicone Products SSP recently received a certificate of compliance COC from NAMSA the worlds leading MedTech Contract Research Organization for a 10-durometer silicone test article that passed USP Biological Reactivity Tests In Vivo for USP Plastics Class VI. Testing was performed by Pacific BioLabs on September 16 2015 in compliance with the standards published in the USP Biocompatibility Testing standards USP. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal.
7 USP Class VI materials EPDM silicone fluorocarbon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600. Food and Drug Administration FDA. Phone 845 855-1000 800 431-0101 Fax 845 855-1139.
What is ADI-Free BSE-Free TSE-Free. USP Class Testing standards are determined by the United States. When production of the elastomer contain no ADI with respect to source manufacture and treatment they cannot.
Many plastics manufacturers find it advantageous to have their materials classified especially if their plastic resins are a likely candidate to be used in medical devices. The United States Pharmacopeia and National Formulary USP-NF determine the USP Class. There are six classes VI being the most rigorous.
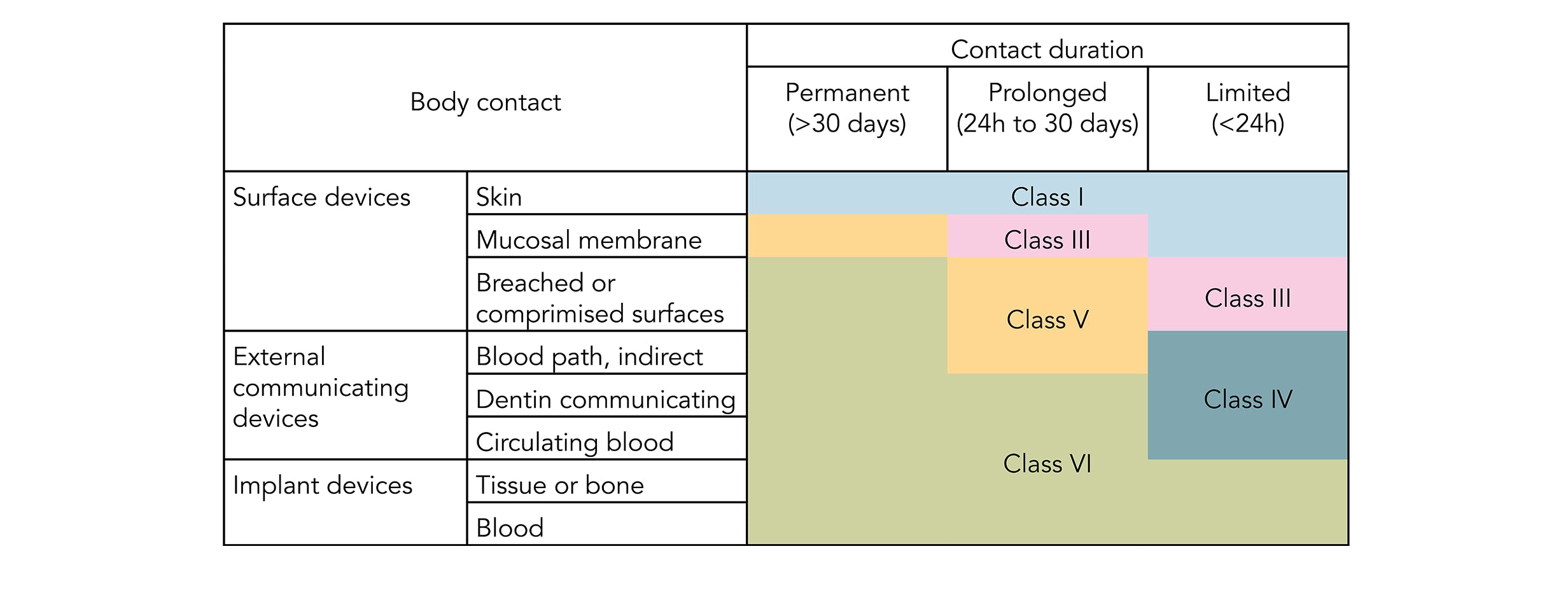
USP Class VI refers to a set of biocompatibility testing requirements from the US. Pharmacopoeia Class VI judges the suitability of plastic material intended for use as containers or accessories for parenteral preparations. Pawling Engineered Products Inc.
Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements. All these special grade products have passed this rigorous test. One standard often overlooked but usually published alongside USP Class VI is FDA 21 CFR 1772600.
Pharmacopeia USP a non-profit organization whose standards inform decision-making at the US. CLASS VI TEST USP Test Article Watershed 11122XC Author Christopher Parker MS. Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant.
Consumers implicitly rely upon the standards put into place by governing agencies to protect the publics health and well-being.

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Usp Class Vi Compliant O Rings Store Eastern Seals Uk Supplier Of Quality Sealing Products

Cpc Usp Class Vi Compliant Polysulfone High Flow Quick Disconnect Couplings Cole Parmer

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

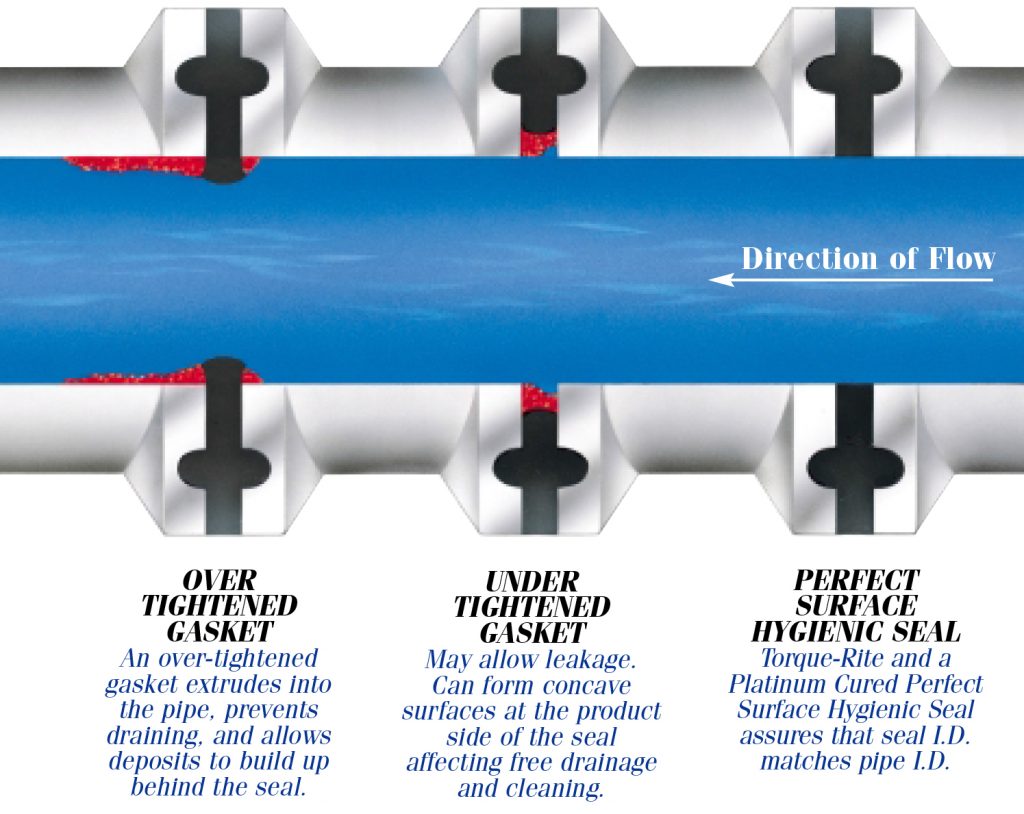
Usp Class Vi Gaskets Newman Sanitary Gasket Company